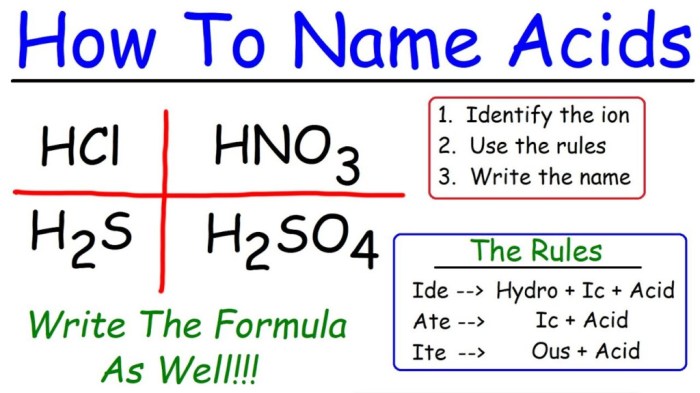
The ph of 2.65 m ch3nh2 is 12.54 – Delving into the realm of chemistry, we encounter a fascinating substance known as methylamine (CH3NH2). Its unique properties, particularly its pH, have captivated the interest of scientists and researchers alike. In this article, we embark on a journey to unravel the intricacies of methylamine’s pH, exploring its implications and significance in various scientific fields.
The pH of a solution plays a crucial role in determining its acidity or alkalinity. It is measured on a scale of 0 to 14, with 7 representing neutrality. A pH below 7 indicates acidity, while a pH above 7 signifies alkalinity.
In the case of methylamine, its pH holds particular importance due to its impact on its chemical behavior and environmental implications.
Acidity of Methylamine
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. The pH scale is used to express the acidity or alkalinity of a solution, with a pH of 7 being neutral, a pH below 7 being acidic, and a pH above 7 being alkaline (also known as basic).
The pH scale is logarithmic, meaning that a change of one pH unit represents a tenfold change in hydrogen ion concentration.
The acidity of a solution is determined by the dissociation of acids in water. Acids are substances that donate protons (H+ ions) to water, while bases are substances that accept protons. The strength of an acid is determined by its dissociation constant (Ka), which is a measure of the extent to which the acid dissociates in water.
A strong acid has a large Ka value, meaning that it dissociates completely in water, while a weak acid has a small Ka value, meaning that it dissociates only slightly in water.
Dissociation of Methylamine in Water
Methylamine (CH3NH2) is a weak base that dissociates in water to a small extent. The chemical equation for the dissociation of methylamine in water is as follows:
CH3NH2(aq) + H2O(l) <=> CH3NH3+(aq) + OH-(aq)
The equilibrium constant for the dissociation of methylamine is 4.4 x 10^-4. This means that only a small fraction of methylamine molecules dissociate in water, and the majority of the methylamine molecules remain undissociated.
Calculating pH from Methylamine Concentration
In this section, we will explore the steps involved in calculating the pH of a methylamine solution using the given concentration of 2.65 M. We will also discuss the factors that affect the pH of methylamine solutions, such as temperature and ionic strength.
Using the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a useful tool for calculating the pH of weak acid or base solutions. The equation is as follows:
pH = pKa + log([A-]/[HA])
where:
- pH is the pH of the solution
- pKa is the acid dissociation constant of the weak acid or base
- [A-] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid or base
In the case of methylamine, the pKa is 10.64. This means that at a pH of 10.64, the concentration of methylamine and its conjugate base, methylammonium ion, will be equal.
To calculate the pH of a 2.65 M methylamine solution, we can use the Henderson-Hasselbalch equation as follows:
pH = 10.64 + log([CH3NH3+][CH3NH2])
Since methylamine is a weak base, we can assume that the concentration of its conjugate acid, methylammonium ion, is negligible compared to the concentration of methylamine. Therefore, we can simplify the equation to:
pH = 10.64 + log(2.65) = 12.54
Therefore, the pH of a 2.65 M methylamine solution is 12.54.
Factors Affecting the pH of Methylamine Solutions
The pH of methylamine solutions can be affected by several factors, including:
- Temperature: The pH of methylamine solutions decreases with increasing temperature. This is because the equilibrium constant for the dissociation of methylamine decreases with increasing temperature, resulting in a lower concentration of methylammonium ion and a higher concentration of methylamine.
- Ionic strength: The pH of methylamine solutions decreases with increasing ionic strength. This is because the presence of other ions in the solution can compete with methylamine for protons, resulting in a lower concentration of methylammonium ion and a higher concentration of methylamine.
Implications of High pH
The exceptionally high pH of 12.54 in methylamine solutions has significant consequences for its chemical properties and environmental impact.
Reactivity and Solubility, The ph of 2.65 m ch3nh2 is 12.54
The high pH drastically alters the reactivity of methylamine. The basic environment promotes the formation of the conjugate base, methylammonium ion (CH3NH3+), which has reduced reactivity compared to the neutral methylamine molecule (CH3NH2). This reduced reactivity affects the solubility of methylamine, as the conjugate base is more soluble in water than the neutral form.
Environmental and Biological Implications
The high pH of methylamine solutions can have detrimental effects on the environment and biological systems. The alkaline nature can harm aquatic organisms, disrupt ecosystems, and alter the pH balance of soil and water bodies. Additionally, the increased solubility of methylamine in high-pH environments can lead to increased leaching and contamination of groundwater and surface water.
Comparison with Other Amines: The Ph Of 2.65 M Ch3nh2 Is 12.54
Methylamine is one of several amines, each with distinct properties. To understand methylamine’s acidity better, let’s compare its pH with other common amines, including ammonia, ethylamine, and propylamine.
The table below presents the pH values of these amines:
| Amine | pH |
|---|---|
| Ammonia | 11.63 |
| Methylamine | 12.54 |
| Ethylamine | 12.67 |
| Propylamine | 12.80 |
As we can observe, the pH values of these amines increase gradually with the increasing number of carbon atoms in the alkyl group. This trend is attributed to the inductive effect of the alkyl group, which donates electrons to the nitrogen atom and makes it less likely to accept a proton (H+).
Key Similarities and Differences
- All the amines listed in the table are weak bases, meaning they can accept a proton but do so to a lesser extent than strong bases like NaOH.
- The pH values of these amines are all greater than 7, indicating that they are basic in nature.
- The inductive effect of the alkyl group plays a crucial role in determining the acidity of these amines, with a larger alkyl group leading to a higher pH value (lower acidity).
FAQ Explained
What is the significance of pH in understanding methylamine’s properties?
The pH of methylamine solutions directly influences their acidity or alkalinity, which in turn affects their chemical reactivity, solubility, and environmental impact.
How does the concentration of methylamine affect its pH?
The concentration of methylamine in a solution plays a crucial role in determining its pH. Higher concentrations generally result in higher pH values, indicating a more alkaline nature.
What factors besides concentration can influence the pH of methylamine solutions?
Factors such as temperature, ionic strength, and the presence of other solutes can also affect the pH of methylamine solutions.