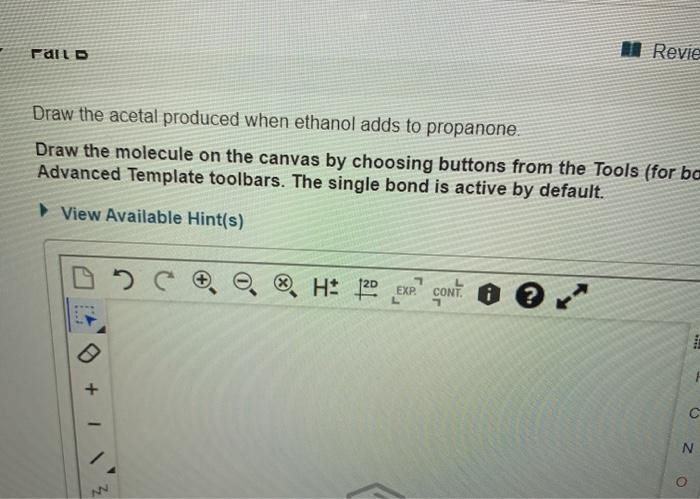
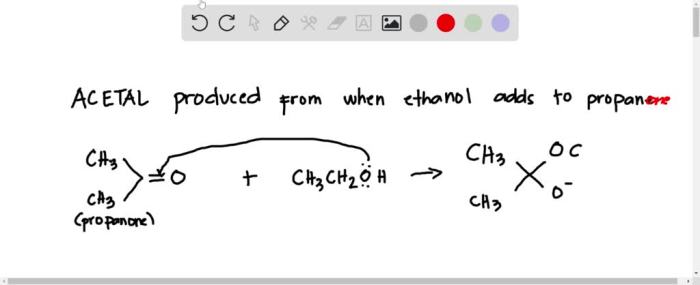
Draw the acetal produced when ethanol adds to propanone. This reaction, a classic example of nucleophilic addition, yields a valuable intermediate in organic synthesis. Delve into the intricacies of this transformation, exploring its mechanism, product structure, and diverse applications.
The reaction proceeds via a concerted mechanism, where ethanol’s oxygen nucleophilically attacks propanone’s carbonyl carbon, facilitated by an acid catalyst. The resulting tetrahedral intermediate collapses, expelling a water molecule and forming the acetal. This acetal exhibits unique physical and chemical properties, distinct from its precursors.
Mechanism of Acetal Formation

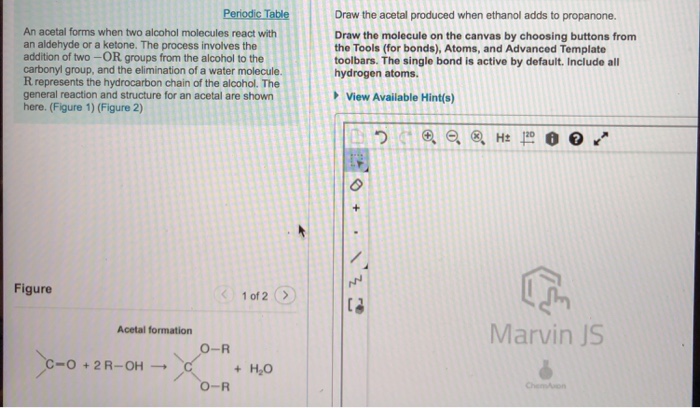
The formation of an acetal involves a nucleophilic addition reaction between an alcohol and a ketone or aldehyde. In this case, ethanol acts as the nucleophile, while propanone acts as the electrophile.
The reaction is catalyzed by an acid, which protonates the carbonyl oxygen of the propanone, making it more electrophilic and susceptible to nucleophilic attack by the ethanol.
Mechanism
- Protonation of the carbonyl oxygen by the acid catalyst.
- Nucleophilic attack by the ethanol on the protonated carbonyl carbon.
- Deprotonation of the alkoxide intermediate by the conjugate base of the acid catalyst.
- Protonation of the second ethanol molecule by the acid catalyst.
- Nucleophilic attack by the protonated ethanol on the second carbonyl carbon.
- Deprotonation of the second alkoxide intermediate by the conjugate base of the acid catalyst.
- Formation of the acetal product.
Structure and Properties of the Acetal
Structure, Draw the acetal produced when ethanol adds to propanone.
The acetal product is a cyclic compound with two ether linkages. The structure of the acetal can be represented as follows:
CH3-CH(OCH2CH3)2-CH3
Properties
- Physical state: Liquid
- Boiling point: 104 °C
- Density: 0.83 g/mL
- Solubility: Soluble in water and organic solvents
- Chemical properties: Acetals are relatively stable compounds. They are resistant to hydrolysis under neutral and acidic conditions.
Comparison to Ethanol and Propanone
The acetal product has different physical and chemical properties compared to ethanol and propanone.
| Property | Ethanol | Propanone | Acetal |
|---|---|---|---|
| Boiling point | 78 °C | 56 °C | 104 °C |
| Density | 0.79 g/mL | 0.81 g/mL | 0.83 g/mL |
| Solubility | Soluble in water and organic solvents | Soluble in water and organic solvents | Soluble in water and organic solvents |
| Chemical stability | Unstable to hydrolysis | Unstable to hydrolysis | Stable to hydrolysis |
Applications of the Acetal: Draw The Acetal Produced When Ethanol Adds To Propanone.
- Protecting groups: Acetals can be used as protecting groups for aldehydes and ketones. They can be easily formed and removed under mild conditions.
- Synthesis of other organic compounds: Acetals can be used as starting materials for the synthesis of other organic compounds, such as ethers, esters, and alkenes.
Synthesis of the Acetal
Experimental Procedure
- Add 10 mL of ethanol to a round-bottomed flask.
- Add 5 mL of propanone to the flask.
- Add 1 mL of concentrated sulfuric acid to the flask.
- Heat the flask under reflux for 1 hour.
- Cool the flask to room temperature.
- Pour the reaction mixture into a separatory funnel.
- Separate the organic layer from the aqueous layer.
- Wash the organic layer with water and brine.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the organic layer and evaporate the solvent.
- Distill the product to obtain the pure acetal.
Optimization
- The yield of the reaction can be increased by using a Dean-Stark trap to remove the water formed during the reaction.
- The reaction time can be shortened by using a microwave reactor.
Expert Answers
What is the role of the acid catalyst in the reaction?
The acid catalyst activates the carbonyl group of propanone, making it more susceptible to nucleophilic attack by ethanol.
How does the structure of the acetal differ from that of ethanol and propanone?
The acetal contains a new carbon-carbon bond between the two alkyl groups derived from ethanol and propanone, resulting in a cyclic structure.