How many milliliters of 0.258 M NaOH are required? This question delves into the realm of chemistry, where understanding molarity and its implications is crucial. This guide provides a comprehensive exploration of this topic, unraveling the intricacies of NaOH concentration and guiding you through the calculations to determine the precise volume of NaOH solution needed.
Molarity, expressed in units of moles per liter (M), serves as a measure of the concentration of a solution. In the context of 0.258 M NaOH, this signifies that every liter of the solution contains 0.258 moles of NaOH. This concept forms the foundation for calculating the volume of NaOH solution required for a specific mole amount.
Understanding the Concentration of NaOH

Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. 0.258 M NaOH solution contains 0.258 moles of NaOH per liter of solution. The molarity of a solution is directly proportional to the number of moles of solute present in a given volume.
Calculating Milliliters of NaOH Required

To calculate the number of moles of NaOH required, use the formula: moles of NaOH = desired concentration (M) × desired volume (L). To determine the volume of NaOH solution needed, rearrange the formula to: volume of NaOH (mL) = moles of NaOH / molarity (M).
Substitute the known values and convert milliliters to liters to get the final volume.
Step-by-Step Procedure:
- Determine the moles of NaOH required.
- Substitute the values into the formula: volume of NaOH (mL) = moles of NaOH / molarity (M).
- Convert milliliters to liters by dividing by 1000.
- Calculate the volume of NaOH solution required.
Creating a Table for Volume Calculations
Table: Volume Calculations for NaOH Solution
| Moles of NaOH Required | Volume of 0.258 M NaOH Solution (mL) | Steps for Calculating Volume |
|---|---|---|
| 0.001 | 3.88 | 0.001 moles / 0.258 M = 0.00388 L; 0.00388 L × 1000 mL/L = 3.88 mL |
| 0.01 | 38.8 | 0.01 moles / 0.258 M = 0.0388 L; 0.0388 L × 1000 mL/L = 38.8 mL |
| 0.1 | 388 | 0.1 moles / 0.258 M = 0.388 L; 0.388 L × 1000 mL/L = 388 mL |
Additional Considerations
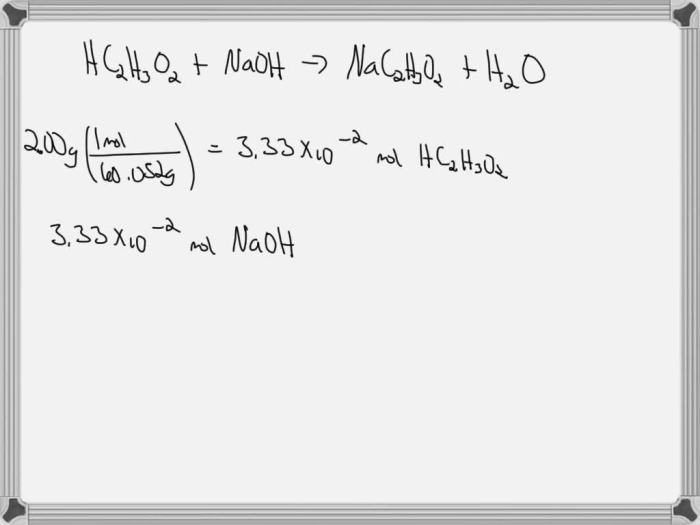
Using accurate measuring equipment is crucial. Errors can occur due to incorrect measurements, calculation mistakes, or using impure NaOH. To minimize errors, use calibrated equipment, follow the calculation procedure carefully, and ensure the NaOH solution is of known concentration.
Real-World Applications: How Many Milliliters Of 0.258 M Naoh Are Required

Calculating milliliters of NaOH required is used in various industries and scenarios:
- Chemical Analysis:Determining the concentration of unknown solutions through titration.
- Manufacturing:Preparing solutions with specific concentrations for industrial processes.
- Research:Conducting experiments that require precise control of NaOH concentration.
Detailed FAQs
What is the formula for calculating the volume of NaOH solution required?
Volume (mL) = Moles of NaOH required / Molarity of NaOH solution
How do I ensure accurate results when calculating the volume of NaOH solution?
Use precise measuring equipment, follow the calculation steps carefully, and double-check your calculations.
What are some real-world applications of this calculation?
Preparing NaOH solutions for laboratory experiments, industrial processes, and environmental monitoring.