Embark on an enlightening journey into the world of acids, where we delve into the fascinating realm of naming acids with the ‘naming acids pogil answer key.’ This comprehensive guide will unravel the mysteries of acid nomenclature, empowering you to conquer the complexities of acid identification with clarity and precision.
From deciphering the IUPAC rules that govern acid naming to exploring the nuances of acid properties and their diverse applications, this guide will illuminate the path to a deeper understanding of these essential chemical compounds.
Acid Nomenclature
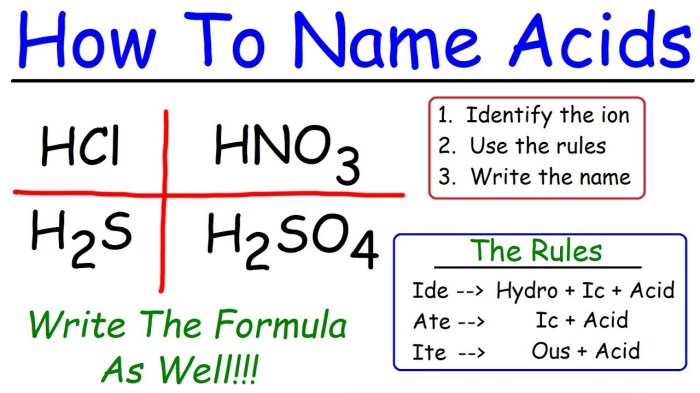
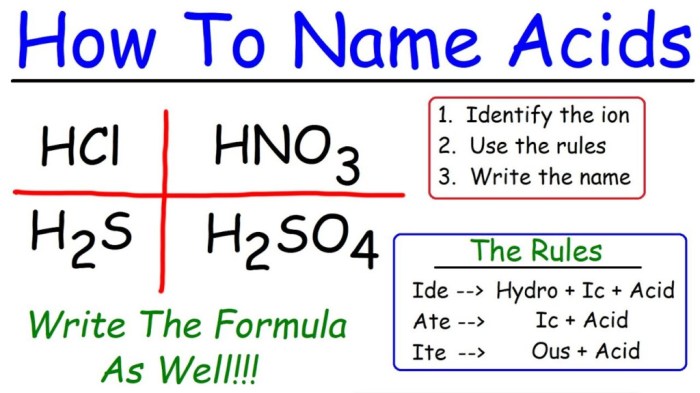
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming acids. These rules are based on the structure of the acid and its chemical composition.
Inorganic Acids
Inorganic acids are acids that do not contain carbon. The IUPAC rules for naming inorganic acids are as follows:
- The name of the acid starts with the word “hydro” followed by the root name of the element that the acid contains.
- The root name of the element is followed by the suffix “-ic” if the element is in its highest oxidation state or “-ous” if the element is in a lower oxidation state.
For example, the IUPAC name for hydrochloric acid is “hydrochloric acid” because it contains hydrogen and chlorine, and chlorine is in its highest oxidation state.
Exceptions to the IUPAC Rules
There are a few exceptions to the IUPAC rules for naming acids. These exceptions include:
- Sulfuric acid (H 2SO 4) is named after the Latin word for sulfur, “sulfur”.
- Nitric acid (HNO 3) is named after the Latin word for nitrogen, “nitrogen”.
- Carbonic acid (H 2CO 3) is named after the Latin word for carbon, “carbo”.
Acid Properties: Naming Acids Pogil Answer Key
Acids are substances that exhibit distinct physical and chemical properties. They are typically characterized by a sour taste, the ability to dissolve certain metals, and a tendency to react with bases.
Acids have a wide range of chemical properties, including their ability to react with bases, metals, and other substances. When acids react with bases, they undergo a process called neutralization, resulting in the formation of a salt and water. Acids can also react with metals, liberating hydrogen gas.
Got the naming acids pogil answer key? Now, let’s venture into the enchanting world of the baobab tree , as portrayed in the beloved classic “The Little Prince.” Just like acids have distinct names, each baobab tree holds a unique story, waiting to be unraveled.
Additionally, acids can act as catalysts in various chemical reactions.
Applications of Acids
Acids have numerous applications in everyday life. They are used in a variety of industrial processes, such as the production of fertilizers, dyes, and plastics. Acids are also used in batteries, food preservation, and the refining of metals.
Acid-Base Titrations
Acid-base titrations are a fundamental technique used in chemistry to determine the concentration of an unknown acid or base. They involve the controlled addition of a known concentration of a base to an unknown concentration of an acid until the reaction is complete.
The purpose of an acid-base titration is to determine the equivalence point, which is the point at which the moles of acid and base are equal. At the equivalence point, the reaction is complete, and the solution is neutral. The volume of base added to reach the equivalence point can then be used to calculate the concentration of the unknown acid.
Steps Involved in Performing an Acid-Base Titration
- Prepare a solution of the unknown acid with a known mass or volume.
- Add a few drops of an indicator to the acid solution.
- Fill a burette with a known concentration of base.
- Slowly add the base to the acid solution, swirling constantly.
- Observe the color change of the indicator, which will indicate the equivalence point.
- Record the volume of base added to reach the equivalence point.
Calculating the Concentration of an Unknown Acid Using Titration Data
The concentration of the unknown acid can be calculated using the following formula:
[Acid] = (Molarity of Base) x (Volume of Base) / (Volume of Acid)
where:* [Acid] is the molarity of the unknown acid
- Molarity of Base is the molarity of the known base
- Volume of Base is the volume of base added to reach the equivalence point
- Volume of Acid is the volume of acid used in the titration
Acid-Base Equilibrium
Acid-base equilibrium is a state of balance in which the forward and reverse reactions of an acid-base reaction occur at the same rate. At equilibrium, the concentrations of the reactants and products remain constant over time.
The equilibrium constant, Keq, is a measure of the extent to which an acid-base reaction proceeds. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium.
Calculating the pH of a Solution Using the Equilibrium Constant
The pH of a solution can be calculated using the equilibrium constant for the dissociation of the acid. The equilibrium constant for the dissociation of a weak acid, HA, is given by:
Ka= [H +][A –]/[HA]
where [H +] is the concentration of hydrogen ions, [A –] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
The pH of a solution can be calculated using the following equation:
pH =
log[H+]
By substituting the equilibrium constant expression into the pH equation, we get:
pH =
log(Ka[HA]/[A –])
Factors that Affect the Position of Acid-Base Equilibrium, Naming acids pogil answer key
The position of acid-base equilibrium is affected by several factors, including:
- The strength of the acid or base:Stronger acids and bases dissociate more completely, resulting in a higher concentration of H +ions and a lower pH.
- The concentration of the acid or base:Increasing the concentration of an acid or base will shift the equilibrium towards the products, resulting in a lower pH.
- The temperature:Increasing the temperature will shift the equilibrium towards the products, resulting in a lower pH.
- The presence of a common ion:Adding a common ion to a solution will shift the equilibrium towards the reactants, resulting in a higher pH.
Questions and Answers
What is the IUPAC nomenclature for acids?
The IUPAC nomenclature for acids follows specific rules to assign systematic names based on their structure and functional groups.
How do you determine the strength of an acid?
The strength of an acid is determined by its ability to donate protons (H+ ions). Stronger acids donate protons more readily.
What is the difference between a monoprotic and a polyprotic acid?
Monoprotic acids can donate one proton per molecule, while polyprotic acids can donate multiple protons per molecule.